Abstract
Fungal diseases in fish cause economic losses all over the world, and knowledge about them is scarce and outdated in Aswan Governorate, Egypt, making interpretation, prevention, and treatment difficult. The necessity to find a fungicide that is natural, environmentally friendly, and does not emerge drug resistance is a must. Therefore, the current study aimed to isolate and diagnose fungal infection in farmed Oreochromis niloticus, causing mortalities, in Aswan Governorate. During 2021, 200 fresh O. niloticus samples were collected from the Sahary Fish Hatchery and Aswan General Authority for Fish Resources Development fish farm. Some fish showed hemorrhagic lesions all over the body, detachment of scales, and fin erosion. Collected tissue samples were cultured on potato dextrose agar and Sabouraud dextrose agar for phenotypic characterization. Macroscopic and microscopic analyses were used to identify the isolated fungi. A total of 18 fungal species and two varieties appertaining to ten fungal genera were recovered from 48 samples out of 200 examined O. niloticus (24%), with Aspergillus flavus being the most prevalent at a rate of 25.6%. The isolated A. flavus was proven to be pathogenic to farmed O. niloticus, as by experimental infection. The natural herb Persicaria salicifolia had an LC50 value of 41.68 mg/l in exposed O. niloticus and was used to treat A. flavus-infected O. niloticus. It can be concluded that A. flavus poses a major hazard to O. niloticus aquaculture and can be treated with 40 mg/kg in feed or 20 mg/l in water of P. salicifolia for 6 days.
Similar content being viewed by others
Introduction
Fish aquaculture is a major source of national income in Egypt and has grown significantly over the last few decades, producing 1,585,839 tonnes in 2021 (FAO 2023; Nasr-Allah et al. 2020; Economic Affairs Sector 2021; Rossignoli et al. 2023). Egypt leads the African continent in fish farming and ranks third internationally in tilapia production behind China and Indonesia, with 971,263 tonnes produced in 2021 (Shaalan et al. 2018; FAO 2023; Economic Affairs Sector 2021).
The resilient Nile tilapia, O. niloticus, is one of the most often used fish species for aquaculture today, and because of its unique biological characteristics, which allow it to live in different environments and aquaculture systems, as well as scientific advances in knowledge of everything related to it, including diseases, culture methods, breeding, genetics, marketing, and consumer demand for it, all of these factors have contributed to its success (El-Sayed 2006; Hamouda and Abd Alkareem 2021; Hamouda and Younis 2021; El-Sayed and Fitzsimmons 2023).
Despite the success of Egypt’s tilapia sector, it faces a number of challenges, including parasitic, bacterial, viral, and fungal diseases, which threaten the industry’s long-term sustainability (Hamouda et al. 2019; Hamouda and Younis 2021; MacKinnon et al. 2023).
Aspergillomycosis is a systemic fungal disease caused by Aspergillus species, specifically A. flavus and A. parasiticus, which produce aflatoxins, the most hazardous and prevalent pollutants in fish feed (Mohamed et al. 2017; Ibrahim 2020). Aflatoxin, which has hepatotoxic, nephrotoxic, neurotoxic, carcinogenic, and immunosuppressive properties in addition to hemorrhagic and causing dermatitis in humans, is produced primarily by A. flavus (Richard 2007; Pitt and Hocking 2009; Oliveira and Vasconcelos 2020; Zahran et al. 2020).
The potential threat posed by the emergence of drug resistance in many fish diseases, as well as the accumulation of toxic residues in fish flesh, increases the risk of environmental pollution. All the aforementioned reasons highlight the need for alternative natural solutions to control these fish pathogens (Gabriel 2019; Hamouda et al. 2019; Mostafa et al. 2020).
Hydrophytes are abundant in antimicrobial phytochemical compounds, and some of these compounds have antifungal properties (Haroon 2006; Maneemegalai and Naveen 2010; Sharma et al. 2012; Altemimi et al. 2017). P. salicifolia, which grows in the Nile Delta as a helophyte and geophyte, has an essential role in complementary medicine and can be used as a natural substitute for traditional fungicides (Boulos 2005; Hussain et al. 2010; Shaltout et al. 2010; Chakma et al. 2018).
So the current study was conducted to explore fungal infection in cultured O. niloticus, causing mortalities, in Aswan Governorate, Egypt, for the first time in Sahary Fish Hatchery and Aswan General Authority for Fish Resources Development fish farm, recording the clinical signs, postmortem lesions, and prevalence of infection. The pathogenicity and histological alterations of the most commonly isolated fungus (A. flavus) to O. niloticus were investigated. As an alternative to standard fungicides, the natural herb P. salicifolia was used to treat A. flavus-infected O. niloticus. The data gathered will serve as the basis for a pathogenic A. flavus control strategy in this region.
Material and method
Study area and collection of fish samples
Between April and October 2021, 200 fresh O. niloticus samples were collected at random, with body weights ranging from 11 to 100 g and total lengths ranging from 8 to 20 cm. The fish were obtained from the Sahary Fish Hatchery and the Aswan General Authority for Fish Resources Development fish farm, Aswan Governorate, Southern Egypt (Fig. 1), which had an archive of rising mortality to reach its peak and then decline. From each location, 100 fish were transported to the Faculty of Fish and Fisheries Technology laboratory at Aswan University for mycological, parasitological, and bacteriological analysis in sterile plastic bags (2/3 oxygen, 1/3 water, and fish), according to Eissa (2016).
Fish for experimental design
A total of 450 O. niloticus, each weighing 50 ± 5 g, were delivered to the wet lab of the Fish Diseases Department at the Faculty of Fish and Fisheries Technology from ponds separate from those showing clinical signs and mortalities of the same hatchery. Prior to the start of the experimental tests, the fish were housed for 14 days in well-prepared glass aquariums containing dechlorinated tap water with a 30-l capacity and continuous air supply at room temperature for acclimatization and inspections of random samples to ensure their clearance of any natural infection. Fish were fed twice daily at a rate of 3% of its body weight. Dead fish were picked up immediately from the glass aquarium to avoid deterioration of water. Samples of fish were examined to exclude infections.
Clinical and postmortem examinations
According to Noga (2010) and the AVMA’s (American Veterinary Medical Association 2020) guidelines, the fish underwent clinical evaluations and were euthanized in the lab. The collected fish were clinically examined to detect any visible changes or clinical abnormalities, and postmortem examination of the internal organs was performed.
Mycological analysis
Fish was washed in running water to remove sediments. For culturing of fungal specimens, two types of media including potato dextrose agar (PDA) and Sabouraud dextrose agar (SDA) were prepared and chloramphenicol (500 mg/l) was supplemented to each preparation of media to avoid bacterial contamination. All of the studied fish’s body surfaces were cleaned by dipping them in 1% formaldehyde for 1 to 5 min, then in 70% alcohol, then in distilled water, where they were properly rinsed (Iqbal et al. 2012). Different parts of fish including the skin, head, gills, fins, liver, and kidney were sliced. The six pieces were inoculated onto both media; isolation was performed in a laminar flow air cabinet. For each medium, three plates from each sample were used. For 5 to 7 days, the inoculated plates were incubated at 28 °C, periodically monitored, and the growing fungus species were re-cultured on Sabouraud dextrose agar with cycloheximide (500 mg/l) to inhibit saprophytic fungi (Acharya and Hare 2022). After that, fungal species were inspected and identified.
Parasitological and bacteriological analysis
The collected samples from the two areas of study were also subjected to parasitological and bacteriological examinations, according to Eissa (2016) and Austin and Austin (2012), respectively, to precisely identify the causative agent or agents of deaths.
Identification of isolated fungal species
Colony morphology, including growth appearance, growth rate, the surface colony texture and color, and the reverse side of the colonies, was observed, as well as microscopic features including fine hyphae morphology (aseptate or septate), conidia, and phialide shapes, which were also used to characterize the pure fungus according to Raper and Fennell (1965), Al-Doory (1980), and Pitt and Hocking (2009). A wet mount preparation of mold was performed by depositing a little bit of a fungus colony on a clean glass slide with a drop of distilled water, covering it with a clean cover slide, and examining it under a microscope. Staining a little bit of the periphery of a fresh colony with lactophenol cotton blue was also used to confirm the identification of isolated mold.
Pathogenicity of A. flavus
The spore suspensions of A. flavus isolated from O. niloticus were prepared for experimental infection according to Willoughby (1994); each pure cultures of A. flavus plate received 20 ml of sterile distilled water to collect the conidial material, which was then collected in sterile tubes. The suspension was filtered using two layers of sterile gauze. The conidial suspension in sterile distilled water was calculated and adjusted to be 9 × 104 spores/ml using an erythrocyte counting chamber of a hemocytometer (Refai et al. 2010; Mohamed et al. 2017). The experiment involved a total of 90 acclimated O. niloticus, which were divided into three equal groups (n = 30 fish per group) and then into three replicates of ten fish each. The first group served as the control (untreated), the second group received sterile saline as a sham injection, and the third group received 0.2 ml of A. flavus spores (9 × 104 spores/ml) intraperitoneally. The clinical symptoms and morality rate of these fish were subsequently recorded daily for the following 7 days.
Fish that showed abnormal clinical signs were used to re-isolate the causative agent mycologically by inoculating specimens from their livers, gills, and musculatures at the injection site into SDA. Gills, liver, and kidney samples were taken from all fish groups at the end of the experiment and kept in 10% neutral buffered formalin for histological analysis.
Preparation of P. salicifolia plant extract
The aquatic plant P. salicifolia was gathered from the banks of the Nile River in Aswan, and its extraction was done using the technique outlined by Mostafa et al. (2020).
The antifungal effect of P. salicifolia extract against pathogenic A. flavus by the Poisoned Food Method
The ethyl acetate extract of P. salicifolia was added to Petri dishes with molten agar at the varying concentrations (10 mg/ml, 20 mg/ml, 40 mg/ml, and 60 mg/ml) and thoroughly stirred. After an overnight pre-incubation, an inoculation can be made using a mycelia disc of A. flavus that is deposited in the plate’s center (0.5 cm in diameter). The diameters of fungal growth in the control and sample plates are measured after additional incubation under conditions appropriate for the tested fungus, and the antifungal effect is evaluated using the method below (Singh and Tripathi 1999; Balouiri et al. 2016):
where Dc is the growth’s diameter in the control plate and Ds is the measurement of the growth diameter in the treated plate.
Toxicity bioassays of P. salicifolia ethyl acetate extract on O. niloticus
According to Essien-Ibok (2020), a toxicity experiment was conducted to evaluate the toxicity of the ethyl acetate extract of P. salicifolia on O. niloticus. In order to pinpoint the exact amount of toxicity, different P. salicifolia concentrations (20 mg/l, 40 mg/l, and 60 mg/l) were used in the experiment. Each of these concentrations was evenly distributed throughout 20 l of water. The experiment was carried out using 120 acclimated O. niloticus from the experimental fish. They were split into four equal groups, each with 30 fish. Each group was split up into three replicas, each with ten fish. As a control, the first group received no extract; the second group received 20 mg/l of P. salicifolia; the third group received 40 mg/l of P. salicifolia; and the fourth group received 60 mg/l of P. salicifolia. Over a period of 24 to 96 h, we monitored the fishes’ clinical symptoms and mortality rates. Logistic regression was used to determine the 96 h LC50 (50% lethal dose).
Effect of P. salicifolia on O. niloticus infected with A. flavus, whether in water or feed
Six groups, each with 30 fish, were created from 180 O. niloticus fish. Each group was split up into three replicas, each with ten fish. Group 1 contains non-treated non-infected fish (control negative), group 2 contains infected non-treated fish (control positive); fish in groups 3 and 4 are treated with plant extracts at concentrations of 20 and 40 mg/l of water, respectively; fish in groups 5 and 6 are treated with plant extracts at concentrations of 20 and 40 mg/kg of feed, respectively. The experiment was monitored for a period of 6 days for clinical signs and mortality rates. Daily water exchanges accounted for around 20% of the aquaria’s total volume; in the aquaria where plant extract was introduced to the water, the equivalent amount of plant extract was added to replace any that was lost. The fish feed twice daily as 3% of the fish biomass. The experimental diet incorporate plant extract in this trial was a commercial normal fish diet that contained 35% crude protein, 5.8% fat, 3.5% crude fiber, and 4100 kcal of digestible energy. The ethyl acetate extract of P. salicifolia was added to the diet at concentrations of 20 mg/kg and 40 mg/kg before the diet was pelletized.
Histopathological examination
At the end of experiment bioassays and treatment trials, different tissue samples were cut and preserved in 10% neutral buffered formalin from the liver, gills, and kidneys of all groups. Following standard processing, the sections were cut into 5-µm-thick sections, fixed in paraffin blocks according to Suvarna et al. (2019), and stained with hematoxylin and eosin for histological analysis (Roberts 2001).
Statistical analysis
According to Duncan (1955), the statistical significance (P < 0.05) of the varied concentrations of the P. salicifolia extracts on A. flavus was determined using one-way analysis of variance (ANOVA) in SPSS 22.0 (SPSS version 22, SPSS Inc., IL, USA), and with the aid of the Excel program, a regression analysis was conducted to assess the association between the inhibition zone and the varied plant extract concentrations.
Results
The naturally infected fish with different fungal species showed hemorrhagic regions, scale detachment, fin erosions, and, in rare instances, ocular opacity and dark body colorations. During postmortem examinations, congested gills, enlarged gall bladder, inflated intestine, ascites, clogged kidneys and spleen, and enlarged liver with hemorrhagic spots were detected.
A total of 18 fungal species and 2 varieties appertaining to 10 fungal genera were recovered from 48 samples of 200 tested O. niloticus, representing a 24% overall prevalence (Table 1).
Many cases of mixed infections in the same fish were detected. Among the recovered fungi, A. flavus (280 colonies) were the most prevalent fungal species (Table 1).
The isolated fungal genera from different organs of O. niloticus were displayed, with the gills being the most affected and the kidney being the least affected (Table 2).
Phenotypic analysis of A. flavus colonies revealed that they appeared white on the second day of inoculation, and as they age, their color changes from pale brown to yellowish green or olive green. Under the microscope, either wet or stained with lactophenol cotton blue, the vesicles were large and spherical, the stigmata were biseriate, loose, and radiating, carrying ovoid, rough conidia, and the conidiophores were long and rough (Fig. 2).
A Yellowish-green colonies of Aspergillus flavus isolated from Oreochromis niloticus after 7 days at 28°C on Sabouraud dextrose agar (SDA). B Olive green colonies of Aspergillus flavus isolated from Oreochromis niloticus after 7 days at 28 °C on potato dextrose agar (PDA). C and D Light microscopic characteristics of wet-mounted (C) and stained with lactophenol cotton blue (D) specimens of Aspergillus flavus isolated from Oreochromis niloticus showing long and rough conidiophores and characteristic head with large and spherical vesicle, biseriate stigmata, and ovoid rough conidia
The fish that had been intraperitoneally injected with A. flavus isolated from naturally infected fish to test its pathogenicity displayed the same clinical symptoms as naturally infected fish, including fin erosions, hemorrhages on the body surface, scale detachment, gill discoloration, rapid opercular movements near the aquarium’s surface, sluggish movement just before death, and mild to moderate ascites. Additionally noted were liver, kidney, spleen, and intestinal congestion (Fig. 3). After 2 days of infection, the fish started to perish, and by the sixth day, mortality had reached 30%. A. flavus re-isolated from the organs of the injected fish. No clinical symptoms or moralities were recorded in the control or sham groups.
By parasitological and bacteriological examinations, no pathogenic parasites or bacteria were recorded from the examined O. niloticus.
The P. salicifolia ethyl acetate extract was effective against A. flavus in vitro (Fig. 4). Concentrations were statistically significant (P < 0.05), indicating that fungus inhibition increases with a corresponding increase in concentration. The extract concentrations (20 mg/ml, 40 mg/ml, and 60 mg/ml) demonstrated dose-dependent inhibition percentages (34.1%, 43.1%, and 65.9%, respectively) (Fig. 5), whereas 10 mg/ml of the extract had no activity.
In order to determine the effects of extended exposure of tilapia to the extract, a toxicity test of an ethyl acetate extract of P. salicifolia was conducted. Fish rose as controls in water without extract showed no signs or deaths. Fish exposed to extract concentrations of 20 mg/l or 40 mg/l did not exhibit any toxicity; however, fish subjected to a concentration of 60 mg/l had severe toxicity. In addition to having missing scales and a black appearance, the poisoned fish was struggling for air close to the aerator. All of the fish had died by the conclusion of the 96-h period as they had fallen to the tank floor with raised fins as the time went. After death, the fish’s postmortem examination revealed rosy gills, a gas-filled intestine, a swollen gallbladder, a hemorrhagic liver, and a congested kidney (Fig. 6). A LC50 value of 41.68 mg/l is established for the extract in the exposed fish (Fig. 7).
Oreochromis niloticus exposed to 60 mg/l of Persicaria salicifolia extract. A One fish showed respiratory distress, gasping and surfacing, and the other one settled down on the aquarium floor. B The fish settled down on the aquarium floor with erected fins. C Rosy gills, a gas-filled intestine (arrow), a distended gall bladder (star), and enlarged liver
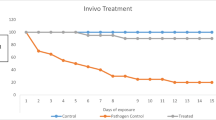
In vivo treatment with an ethyl acetate extract of P. salicifolia against A. flavus infection was performed to control the disease in O. niloticus. The least amount of mortality (10%) across all groups was achieved by group 6 (40 mg/kg in feed), which was followed by group 3 (20 mg/l in water) with 13.33% mortality (Table 3, Figs. 8 and 9).
According to histopathological examination conducted during the experiment bioassays and the treatment trial with ethyl acetate extracts of P. salicifolia on O. niloticus, the control group did not exhibit any abnormalities in all organs, e.g., gills and kidneys (Figs. 10 and 11A). The extract at a dosage of 20 mg/l did not exhibit any toxicity and this appeared in the normal histoarchitecture of all organs, e.g., the kidney and liver (Figs. 11C and 12A). Fish exposed to the extract concentration of 40 mg/l displayed diffuse epithelial hyperplasia of the primary lamellae (Fig. 10B). Fish subjected to a concentration of 60 mg/l of the extract showed severe toxicity as large necrotic areas in liver (Fig. 12B). Fish infected with A. flavus and left untreated displayed necrosis of the renal tubules associated with the presence of the fungus spores, and infiltration of inflammatory cells (Fig. 11B). Fish infected with A. flavus and treated with 20 mg/l of the P. salicifolia extract developed focal adhesion of the secondary lamellae, hyperplasia of the lamellar epithelium with infiltration of inflammatory cells (Fig. 10C), and mild to moderate hydropic degeneration of the hepatic cells (Fig. 12C). Fish infected with A. flavus and treated with 40 mg/l of the P. salicifolia extract displayed multi-focal necrotic areas within renal tubules (Fig. 11D). A. flavus-infected fish that were fed the P. salicifolia extract at a dose of 40 mg/kg displayed focal hyperplasia of the lamellar epithelium and epithelium lifting of a few secondary lamellae, and a focal necrotic area of the pancreatic acini (Figs. 10 and 12D).
Representative photomicrograph of H&E-stained gills of Oreochromis niloticus. A Control group shows normal histoarchitecture consisted mainly of primary filaments (PL) branching out into tiny secondary filaments (SL). B Fish group exposed to Persicaria salicifolia extract at concentrations of 40 mg/l shows diffuse hyperplasia of the covering epithelium of the primary lamellae (arrow). C Gills of a fish group infected with Aspergillus flavus challenged with 20 mg/l of the Persicaria salicifolia extract show focal adhesion of the secondary lamellae associated with hyperplasia of the lamellar epithelium and infiltration of inflammatory cells (arrowhead). D Gills of the fish group infected with Aspergillus flavus fed on a normal diet supplemented with Persicaria salicifolia extract at a concentration of 40 mg/kg show focal hyperplasia of the lamellar epithelium (arrowhead) and epithelium lifting of a few secondary lamellae (arrow). Scale bar = 50 µm
Representative photomicrograph of H&E-stained posterior kidney of Oreochromis niloticus. A The control fish group shows normal histoarchitecture consisted mainly of renal tubules (RT). B Fish group infected with Aspergillus flavus shows necrosis of renal tubules (white arrowhead) associated with presence of the spores of the fungus (black arrowheads) and infiltration of inflammatory cells (arrow). C Fish group exposed to Persicaria salicifolia extract at concentrations of 20 mg/l shows normal histoarchitecture consisting mainly of renal tubules (RT). D Fish group exposed to Persicaria salicifolia extract at concentrations of 40 mg/l shows multi-focal necrotic areas within renal tubules (arrowheads). Scale bar = 50 µm
Representative photomicrograph of H&E-stained hepatopancreas of Oreochromis niloticus. A Fish group exposed to Persicaria salicifolia extract at concentrations of 20 mg/l shows normal histoarchitecture, where hepatocyte cords (H) and pancreatic acini (PA) surround central veins. B Fish group exposed to Persicaria salicifolia extract at concentration 60 mg/l shows large necrotic area (asterisk). C Fish group infected with Aspergillus flavus challenged with 20 mg/l of Persicaria salicifolia extract shows mild to moderate hydropic degeneration of the hepatic cells (arrows). D Fish group infected with Aspergillus flavus fed on a normal diet supplemented with Persicaria salicifolia extract at a concentration of 40 mg/kg shows focal necrotic area of the pancreatic acini (arrowhead). Scale bar = 50 µm
Discussion
This is the first investigation on fungal infections in cultured O. niloticus in Egypt’s Aswan Governorate; previous studies were carried out on wild fish by El-Zayat (1988, 2000).
The clinical signs and postmortem lesions recorded during the examination of the naturally infected O. niloticus with fungi especially A. flavus could be brought on by toxic metabolites, lipase and protease enzymes produced by these fungi (Olufemi 1985; Moharram and El-Zayat 1989; Iqbal and Saleemi 2013; Hashem et al. 2020, Zakaria et al. 2021; Mahboub et al. 2022).
The colony morphology of A. flavus was exactly the same as that reported by many other prior researchers (Abd El-Tawab et al. 2020; Hashem et al. 2020; Zakaria et al. 2021; Mahboub et al. 2022). Depending on the culture media employed in their culture, the colonies of Aspergillus flavus and other Aspergillus may have different colors (Brun et al. 2001).
In our study, A. flavus was the most frequently isolated mold (25.6%); this result was in line with the findings of Refai et al. (2010) and Abd El-Tawab et al. (2020). The isolated A. flavus from naturally infected O. niloticus was proven to be pathogenic to other non-infected fish (Abdel Monem et al. 1995; Eissa et al. 2022) with 30% morality by the end of the sixth day of infection. It was then re-isolated from the morbid fish indicating its pathogenicity to O. niloticus. This species was believed to produce protease and aflatoxins, which may be responsible for the infection (Mohamed et al. 2017). In this regard, Oliveira and Vasconcelos (2020) found that A. flavus is the main producer of aflatoxins, which impair immune function and increase fish mortality (Marijani et al. 2019).
Excessive use of fungicides in the treatment of fish fungal diseases may emerge drug resistance, accumulation of toxic residues in fish flesh, and increase the danger of environmental pollution. According to Mostafa et al. (2020), fungicides are known to possess numerous carcinogenic and teratogenic properties; hence, the development of fungicides that are both effective and environmentally friendly is crucial. Therefore, P. salicifolia was used in this study as a natural fungicide.
The phytochemical compounds, such as glycosides, flavonoids, and phenolic acids, which have antifungal and antioxidant effects, may be the factor responsible for P. salicifolia’s ethyl acetate extract’s antifungal activity against A. flavus (Haroon 2006; Abd El-Kader et al. 2012; Sharma et al. 2012; Hussein and Mohamed 2013; El-Swaify et al. 2015; El-Anwar et al. 2016). Furthermore, because flavonoids function as cell membrane stabilizers by detoxifying xenobiotics and preventing radical-induced lipid peroxidation, they are well known for their potent free radical scavenging and hepatoprotective effects (Heim et al. 2002; Kinjo et al. 2006; Wilms et al. 2008). This is consistent with Hussain et al. (2010), who reported that the crude extract of Polygonum persicaria leaf showed the highest activity against A. niger and moderate activity against A. flavus, and Chakma et al. (2018), who found that the methanolic extract of P. glabra is effective against A. niger. Furthermore, Maqbool et al. (2022) detected the antifungal activity of P. Barbata against A. niger, and Maksimović et al. (2023) declared that P. amphibia inhibited the growth of Aspergillus spp.
P. salicifolia extract (20 mg/l in water or 40 mg/kg in diet) significantly reduced mortality in infected fish, indicating that P. salicifolia’s extract treats O. niloticus with A. flavus infection.
O. niloticus exposed to 60 mg/l of P. salicifolia extract shown significant toxicity despite the extract’s strong fungicidal activity in vitro; this finding is consistent with the study’s finding that O. niloticus’ LC50 value for P. salicifolia was 41.68 mg/l.
The gills, kidney, and liver of O. niloticus infected with A. flavus displayed necrotic and degenerative changes in the current investigation. The fungus’ toxic metabolites may be the cause of these histological alterations (Abdel Monem et al. 1995; Eissa et al. 2022).
Histopathological analyses on vital organs such as gills, kidney, and liver of O. niloticus infected with A. flavus and treated with different concentrations of P. salicifolia’s extract (20, 40 mg/l, or 20, 40 mg/kg) showed only minor pathological changes; however, these concentrations were still safe in comparison to 60 mg/l, which demonstrated notable cytotoxicity.
Conclusion
According to this study, the most common fungal infection in farmed O. niloticus in the Aswan Governorate, Egypt, is A. flavus (25.6%). Experimental and histological evidence confirm that A. flavus is pathogenic to farmed O. niloticus. The LC50 value of the herbal extract of P. salicifolia in exposed O. niloticus is 41.68 mg/l. Treatment of O. niloticus infected with A. flavus with 40 mg/kg in feed or 20 mg/l in water of P. salicifolia for 6 days is indicated for good survival rates and minor pathological abnormalities at these concentrations. The data gathered will serve as the basis for a pathogenic A. flavus control strategy in this region. Further investigations are required on P. salicifolia’s extract as a promising fungicide for O. niloticus and other fish species.
Data availability
The data sets used in the present study are accessible on reasonable request from the corresponding author.
Abbreviations
- O. niloticus :
-
Oreochromis niloticus
- A. flavus :
-
Aspergillus flavus
- P. salicifolia :
-
Persicaria salicifolia
- FFT:
-
Faculty of Fish and Fisheries Technology
- PDA:
-
Potato dextrose agar
- SDA:
-
Sabouraud dextrose agar
- Dc:
-
The growth's diameter in the control plate
- Ds:
-
The measurement of the growth diameter in the treated plate
- LC50 :
-
50% Lethal dose
- A. fumigatus :
-
Aspergillus fumigatus
- A. nidulans :
-
Aspergillus nidulans
- A. niger :
-
Aspergillus niger
- A. terreus :
-
Aspergillus terreus
- A. parasiticus :
-
Aspergillus parasiticus
- A. ustus :
-
Aspergillus ustus
- E. nidulans var. echinulata :
-
Emericella nidulans var. echinulata
- E. nidulans var. lata :
-
Emericella nidulans var. lata
- PL:
-
Primary filaments
- SL:
-
Secondary filaments
- RT:
-
Renal tubules
- H:
-
Hepatocytes cords
- PA:
-
Pancreatic acini
References
Abd El-Kader AM, Nafady AM, Ahmed AS, Ibraheim ZZ (2012) Antioxidant, hepatoprotective and antimicrobial activities of the aerial parts of Polygonum bellardii. Bull Pharm Sci 35(1):43–54
Abd El-Tawab AA, El-Hofy FI, Moustafa EM, Halawa MR (2020) Insight into isolation, identification and antimicrobial sensitivity of some moulds isolated from fresh water fishes. Adv Anim Vet Sci 8(2):174–182
Abdel Monem N, Afifi SH, El.Allway TA, Ahmed ShM (1995) Clinical and histopathological studies of Aspergillus flavous in Nile tilapia (Oreochromis niloticus). Assiut Vet Med J 34(67):96–108
Acharya T, Hare J (2022) Sabouraud agar and other fungal growth media. In: Gupta VK, Tuohy M (eds) Laboratory protocols in fungal biology. Fungal biology. Springer, Cham
Al-Doory Y (1980) Laboratory medical mycology. Lea and Febiger, Philadelphia
Altemimi A, Lakhssassi N, Baharlouei A, Watson DG, Lightfoot DA (2017) Phytochemicals: extraction, isolation, and identification of bioactive compounds from plant extracts. Plants (basel) 6(4):42. https://doi.org/10.3390/plants6040042
Austin B, Austin DA (2012) Bacterial fish pathogens; diseases of farmed and wild fish (5th ed.) Springer Dordrecht. https://doi.org/10.1007/978-94-007-4884-2
AVMA (American Veterinary Medical Association) (2020) Guidelines for the euthanasia of animals: 2020 Edition, 1931 N. Meacham Road, Schaumburg, IL 60173, USA
Balouiri M, Sadiki M, Ibnsouda SK (2016) Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal 6(2):71–79. https://doi.org/10.1016/j.jpha.2015.11.005
Boulos L (2005) Flora of Egypt, vol 4. Al-Hadara Publishing, Cairo, p 617
Brun S, Bouchara JP, Bocquel A, Basile AM, Contet-Audonneau N, Chabasse D (2001) Evaluation of five commercial Sabouraud gentamicin-chloramphenicol agar media. Eur J Clin Microbiol Infect Dis 20(10):718–723. https://doi.org/10.1007/s100960100577
Chakma U, Shishir TA, Deeba MT, Islam MN, Morshed Z, Islam R et al (2018) Anti-microbial screening and cytotoxic activity: in-vitro analysis of Persicaria glabra. J Pharm Innov 7(4):940–943
Duncan DB (1955) Multiple Range and Multiple F Tests. Biometrics 11(1):1–42. https://doi.org/10.2307/3001478
Economic Affairs Sector (2021) Statistics of fish production, insects and food-manufacturing. Arab Republic of Egypt, Ministry of Agriculture and Land Reclamation, Economic Affairs Sector. https://www.agri.gov.eg/library/24
Eissa AE (2016) Clinical and Laboratory Manual of Fish Diseases. LAPLAMBERT Academic Publishing
Eissa EH, Ezzo OH, Khalil HS, Tawfik WA, El-Badawi AA, Elghany A et al (2022) The effect of dietary nanocurcumin on the growth performance, body composition, haemato-biochemical parameters and histopathological scores of the Nile tilapia (Oreochromis niloticus) challenged with Aspergillus flavus. Aquac Res 53:6098–6111
El-Anwar RM, Ibrahim AS, Abo El-Seoud KA, Kabbash AM (2016) Phytochemical and biological studies on Persicaria salicifolia Brouss. ex Willd growing in Egypt. Int Res J Pharm 7(8):4–12. https://doi.org/10.7897/2230-8407.07889
El-Sayed A-FM, Fitzsimmons K (2023) From Africa to the world—the journey of Nile tilapia. Rev Aquac 15(Supp 1):6–21
El-Sayed AM (2006) Tilapia culture. CABI Publishing, CAB International. https://doi.org/10.1079/9780851990149.0000
El-Swaify ZA, Moaty DA, Youssef MM, El-Hela A (2015) Phytochemical studies on Persicaria salicifolia plant and seeds from Egypt. Azhar Bull Sci 26(2):37–45
El-Zayat SA (2000) Fungi associated with eight species of Aswan High Dam fishes. J Union Arab Biol Microbiol Virus 9B:253–263
El-Zayat SA (1988) Studies on freshwater fungi of Aswan High Dam Lake. Ph.D. Thesis, Bot. Dept. Fac. Sci., Assiut University, Egypt
Essien-Ibok MA (2020) The toxicity of ethanolic extract of Alchornea cordifolia leaf on Clarias gariepinus fingerlings. Int J Sci Res Environ Sci Toxicol 5(1):1–5
FAO (2023) National aquaculture sector overview: Egypt. Fisheries and Aquaculture Department. https://www.fao.org/figis/pdf/fishery/countrysector/naso_egypt/en?title=FAO%20National%20Aquaculture%20Sector%20Overview%20%28NASO%29
Gabriel NN (2019) Review on the progress in the role of herbal extracts in tilapia culture. Cogent Food Agric 5(1):1619651
Hamouda AH, Abd Alkareem OM (2021) Insight into the correlation between parasitic infestation and heavy metal concentrations in tilapia species inhabiting Lake Nasser, Egypt. Aquac Res 52(7):3425–3437
Hamouda AH, Younis AE (2021) Characterization of Clinostomum cutaneum and Clinostomum phalacrocoracis in tilapia species of Aswan Governorate, Egypt: A morphological, molecular and histopathological study. Aquac Res 52(12):6726–6740
Hamouda AH, Moustafa EM, Zayed MM (2019) Overview on the most prevailing bacterial diseases infecting Oreochromis niloticus at Aswan fish hatchery, Egypt. Adv Anim Vet Sci 7(11):950–961
Haroon AM (2006) Effect of some macrophytes extracts on growth of Aspergillus parasiticus. Egypt J Aquat Res 32:301–313
Hashem OM, Zaki VH, Adawy RS (2020) Incidence and molecular characterization of fungi and yeast isolated from cultured catfish and Nile tilapia. Mansoura Vet Med J 21(3):61–66
Heim KE, Tagliaferro AR, Bobilya DJ (2002) Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nutr Biochem 13(10):572–584. https://doi.org/10.1016/s0955-2863(02)00208-5
Hussain F, Ahmad B, Hameed I, Dastagir Gh, Sanaullah P, Azam S (2010) Antibacterial, antifungal and insecticidal activities of some selected medicinal plants of polygonaceae. Afr J Biotechnol 9(31):5032–5036
Hussein SR, Mohamed AA (2013) Antioxidant activity and phenolic profiling of two Egyptian medicinal herbs Polygonum salicifolium Brouss ex Wild and Polygonum senegalense Meisn, Analele Univ. din Oradea. Fasc Biol 20(2):59–63
Ibrahim T (2020) Diseases of Nile tilapia with special emphasis on water pollution. J Environ Sci Technol 13(1):29–56. https://doi.org/10.3923/jest.2020.29.56
Iqbal Z, Saleemi S (2013) Isolation of pathogenic fungi from a freshwater commercial fish, Catla catla (Hamliton). Sci Int (lahore) 25(4):851–855
Iqbal Z, Sheikh U, Mughal R (2012) Fungal infections in some economically important freshwater fishes. Pak Vet J 32(3):422–426
Kinjo J, Hitoshi M, Tsuchihashi R, Korematsu Y, Miyakoshi M, Murakami T et al (2006) Hepatoprotective constituents in plants: protective effects of natural occurring flavonoids and miscellaneous phenolic compounds as determined in a HepG2 cell cytotoxicity assay. J Nat Med 60:36–41
MacKinnon B, Debnath PP, Bondad-Reantaso MG, Fridman S, Bin H, Nekouei O (2023) Improving tilapia biosecurity through a value chain approach. Rev Aquac 15(Suppl. 1):57–91. https://doi.org/10.1111/raq.12776
Mahboub HH, Nada HS, Abdel-Ghany HM, Ghanem R, Ahmed Ismail T, Abdel Rahman AN (2022) Detection, diagnosis, Koch’s postulate, hepatorenal and antioxidant indicators for some systemic pathogenic fungi invading the liver and kidneys of African catfish (Clarias gariepinus) in Egypt with a histopathological approach. Aquac Res 53(7):2670–2685
Maksimović M, Jovanović M, Nikolić B, Tomić N, Tenji D, Stević T et al (2023) Persicaria amphibia, an old traditional remedy and wild edible herb: in vitro evaluation of cytotoxicity and antimicrobial properties. Bot Serb 47(1):1–8
Maneemegalai S, Naveen T (2010) Evaluation of antibacterial activity of flower extracts of Cassia auriculata L. Ethnobot Leafl 14:8–20
Maqbool M, Ajaib M, Ishtiaq M, Anwar R, Hussain T, Mushtaq W et al (2022) Antibacterial and Antifungal activity of Persicaria barbata (L.) Hara (PBH) from district Bhimber (AJK), Pakistan. Biosci Res 19(1):315–321
Marijani E, Kigadye E, Okoth S (2019) Occurrence of fungi and mycotoxins in fish feeds and their impact on fish health. Int J Microbiol 1–17.https://doi.org/10.1155/2019/6743065
Mohamed HMA, Emeish WFA, Braeuning A, Hammad S (2017) Detection of aflatoxin-producing fungi isolated from Nile tilapia and fish feed. EXCLI J 13(16):1308–1318. https://doi.org/10.17179/excli2017-960
Moharram A, El-Zayat SA (1989) Lipase and protease production by fungi isolated from scales of Tilapia nilotica. Bull Fac Sci Assiut Univ 18(I-D):109–117
Mostafa AA, Al-Askar AA, Yassin MT (2020) Anti-saprolegnia potency of some plant extracts against Saprolegnia diclina, the causative agent of saprolengiasis. Saudi J Biol Sci 27(6):14821487
Nasr-Allah A, Gasparatos A, Karanja A, Dompreh E, Murphy S, Rossignoli C et al (2020) Employment generation in the Egyptian aquaculture value chain: implications for meeting the Sustainable Development Goals (SDGs). Aquaculture 520:734940. https://doi.org/10.1016/j.aquaculture.2020.734940
Noga EJ (2010) Fish disease: diagnosis and treatment, 2nd edn. John Wiley & Sons, p 544
Oliveira M, Vasconcelos V (2020) Occurrence of mycotoxins in fish feed and its effects: a review. Toxins (basel) 12(3):160. https://doi.org/10.3390/toxins12030160
Olufemi BE (1985) The Aspergilli as pathogens of cultured fishes. In: Recent advances in aquaculture. Springer, Boston, MA 2:193–218
Pitt JI, Hocking AD (2009) Fungi and food spoilage 3rd edition. Springer Science + Business Media, LLC, Dordrecht- Heidelberg- London, U.K. New York, NY. pp 11–419
Raper KB, Fennell DJ (1965) The genus Aspergillus. Williams and Wilkins, Baltimore
Refai MK, Mohamed LA, Kenawy AM, El-SMA S (2010) The assessment of mycotic settlement of freshwater fishes in Egypt. J Am Sci 6)11):575–600
Richard JL (2007) Some major mycotoxins and their mycotoxicoses: an overview. Int J Food Microbiol 119(1–2):3–10. https://doi.org/10.1016/j.ijfoodmicro.2007.07.019
Roberts RJ (2001) Fish pathology, 3rd edn. W. B. Saunders, Philadelphia, p 472
Rossignoli CM, Manyise T, Shikuku KM, Nasr-Allah AM, Dompreh EB, Henriksson PJG et al (2023) Tilapia aquaculture systems in Egypt: Characteristics, sustainability outcomes and entry points for sustainable aquatic food systems. Aquaculture 577:739952. https://doi.org/10.1016/j.aquaculture.2023.739952
Shaalan M, El-Mahdy M, Saleh M, El-Matbouli M (2018) Aquaculture in Egypt: insights on the current trends and future perspectives for sustainable development. Rev Fish Sci Aquac 26(1):99–110. https://doi.org/10.1080/23308249.2017.1358696
Shaltout KH, Sharaf A, Ahmed DA (2010) Plant life in the Nile Delta. Tanta Univer Press, p 169
Sharma M, Mandloi AK, Pandey Govind, Sahni YP (2012) Antimicrobial activity of some medicinal plants against fish pathogens. Int Res J Pharm 3(4):28–30
Singh J, Tripathi NN (1999) Inhibition of storage fungi of blackgram (Vigna mungo L.) by some essential oils. Flavour Frag J 14(1):1–4
Suvarna SK, Layton Ch, Bancroft JD (2019) Bancroft’s theory and practice of histological techniques, 8th edn. Elsevier Limited, London
Willoughby LG (1994) Fungi and fish diseases, 1st edn. Pisces Press, Stirling
Wilms LC, Kleinjans JC, Moonen EJ, Briedé JJ (2008) Discriminative protection against hydroxyl and superoxide anion radicals by quercetin in human leucocytes in-vitro. Toxicol in Vitro 22(2):301–307. https://doi.org/10.1016/j.tiv.2007.09.002
Zahran E, Risha E, Hamed M, Ibrahim T, Palić D (2020) Dietary mycotoxicosis prevention with modified zeolite (Clinoptilolite) feed additive in Nile tilapia (Oreochromis niloticus). Aquaculture 515:734562
Zakaria Kh, Teet SE, Hamzah NH, Aznan AS, Abdul Manaf MT, Wan Ibrahim WN et al (2021) Isolation and Identification of fungi associated with diseased freshwater fishes in Terengganu, Malaysia. Songklanakarin J Sci Technol 43(4):1131–1139
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). No fund.
Author information
Authors and Affiliations
Contributions
The authors a, b, c and d contributed equally to this work, whereas they designed and conducted the research, and wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethical approval
The Animal Use and Care Committee of the Faculty of Fish and Fisheries Technology at Aswan University in Egypt (Fac. FFT. No. 9/2021) gave its approval for the current study’s standard operating procedure.
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: Amany Abbass
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Fungal infection in cultured Oreochromis niloticus in Aswan Governorate, Egypt, was explored.
• Persicaria salicifolia herbal extract has an LC50 value of 41.68mg/l in exposed O. niloticus.
• The extract of P. salicifolia was utilized as a natural alternative to traditional fungicides for treatment of Aspergillus flavus infection in O. niloticus.
• It is recommended to treat O. niloticus infected with A. flavus with 40 mg/kg in feed or 20 mg/l in water of P. salicifolia for 6 days.
• Further investigations are required on P. salicifolia’s extract as a promising fungicide for O. niloticus and other fish species.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Zayat, S.A., Abdel-Motaal, F.F., Mohamed, S.H. et al. Efficiency of Persicaria salicifolia as a natural alternative antifungal against Aspergillus flavus infection in Oreochromis niloticus from Aswan Governorate, Egypt. Aquacult Int (2024). https://doi.org/10.1007/s10499-024-01417-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10499-024-01417-3